Replimune
@Replimune
Followers
749
Following
7
Media
90
Statuses
156
Igniting a systemic immune response to cancer. Our mission is to transform cancer treatment by pioneering novel oncolytic immunotherapies.
Woburn, MA & Oxford, UK
Joined December 2016
Today, we announced that the FDA has accepted our resubmission of the RP1 BLA for the treatment of advanced melanoma. Read more: https://t.co/xTyZoWpNhY
0
0
5
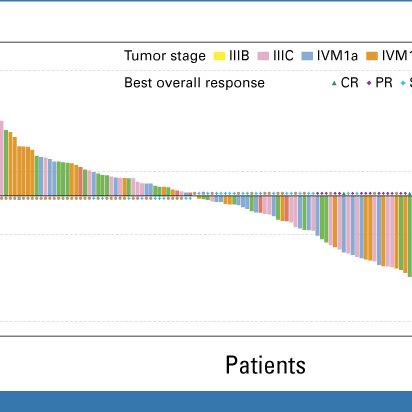
Earlier this week, data from the IGNYTE trial of RP1 combined with nivolumab in anti-PD-1 failed melanoma was published online in the Journal of Clinical Oncology #melanoma Read the publication here:
ascopubs.org
PURPOSEEffective treatment options for melanoma after immune checkpoint blockade failure are limited. RP1 (vusolimogene oderparepvec) is a herpes simplex virus type 1–based oncolytic immunotherapy,...
1
6
9
The MRF Breakthrough Consortium (MRFBC) annual in-person meeting is officially underway at #ASCO2025! This important gathering brings together leaders in melanoma research and clinicians to exchange insights, strengthen collaboration and drive progress in the field. We’re
0
1
2
This morning, we reported our fiscal fourth quarter and year end 2025 financial results, and provided an update on our progress. Read more here: https://t.co/2SMYlncrDh
0
0
1
#NEWS: The FDA accepted the Biologics License Application (#BLA) for our novel oncolytic immunotherapy for the treatment of advanced #melanoma. This milestone is an important step in our efforts to build a skin cancer franchise. Learn more: https://t.co/b4gblBNNk5
0
0
5
Today at #ESMO24 we presented data from the IGNYTE clinical trial of our investigational oncolytic immunotherapy RP1 combined with nivolumab in anti-PD-1-failed melanoma. Learn more about our late-breaking oral presentation here: https://t.co/0yhKHRdDWF
1
3
8
Today, we announced that the first patient has been randomized and dosed in the IGNYTE-3 study, our confirmatory Phase 3 trial of the investigational oncolytic immunotherapy, RP1 in combination with nivolumab in patients with advanced #melanoma. Read more: https://t.co/aqG86N6VWp
0
1
8
In a new @BiotechTVHQ interview with @bradloncar, Replimune CEO Sushil Patel discusses our recent positive, topline data by independent central review of RP1 + nivolumab for anti-PD1 failed melanoma. Watch here: https://t.co/H0a5ZaWCXx
biotechtv.com
Sushil Patel describes Replimune's ASCO and follow-on data from last week in the context of PD-1 response rates and competitor data from Iovance, and discusses Replimune's plans to file for acceler...
0
2
5
Today, we announced a $100M financing, which follows the strong primary analysis data from the RP1 IGNYTE clinical trial in anti-PD1 failed melanoma. Proceeds will enable full commercial scale up to support a potential launch in 2H 2025. Read more: https://t.co/9LmLOo7Hf6
0
1
1
We had an amazing time at #ASCO24! We were honored to present the latest data demonstrating our clinical progress in oncolytic #immunotherapy across our portfolio. Missed our presentations? Our posters and data can be found at https://t.co/mEHoQ1hRDM.
0
1
3
Today, we shared topline primary analysis results by independent central review from our IGNYTE clinical trial of RP1 + nivolumab in anti-PD1 failed #melanoma. Read more: https://t.co/frPniyJ5hl
0
3
7
Today at #ASCO24 we will present RP1 / IGNYTE data in anti-PD-1-failed melanoma and RP2 data in uveal melanoma. Learn more about these two oral presentations here: https://t.co/m45ZODlxQT
0
0
3
While May is coming to an end, our work to improve the lives of patients living with #melanoma continues. This month – and every month – we recognize the individuals and their families who have been impacted by skin cancer.
0
0
1
We are excited to be in Chicago for #ASCO24 to learn all about the latest and greatest advancements in #oncology – don’t miss us at Booth #16143 or at one of our presentations! https://t.co/OltfyA0nmR
0
0
0
Today, Replimune reported fiscal Q4 and year ended 2024 financial results and provided corporate updates: https://t.co/1IXu4XEmMq
0
0
0
Replimune was and continues to be built by amazing leaders and veterans in biotech – we are incredibly thankful for the contributions of our founders/executive team as we prepare for global growth and new milestones. Read more here: https://t.co/p7EOWmjlIm
0
0
2
Today, we announced changes to our executive leadership team designed to support preparations for the commercial launch of RP1, pending regulatory submission and approval in anti-PD1 failed melanoma. Read more here: https://t.co/p7EOWmjlIm
0
0
3
In just a few hours, we will join Dr. Gino from @uscnorris and patient Advocate, Donna Soskin for our Ask the Expert series as they discuss the mechanisms of how oncolytic viruses work against cancer, how these viruses are designed to minimize risk of infection, the patient
1
4
5
This morning, we shared a comprehensive update on RP1. The full announcement is available here: https://t.co/VMaxIsx86E
0
0
3
Today, we present updated data on RP2 in uveal melanoma during a Plenary Session at the 20th International Congress of the Society for Melanoma Research. Read more on our website here: https://t.co/356ubehoxw
1
2
4