Oribiotech
@OriBiotech
Followers
528
Following
243
Media
858
Statuses
1K
Ori Biotech is an innovator in Cell and Gene Therapy manufacturing, delivering scalable solutions to address the critical needs of therapeutics developers.
London and New Jersey
Joined December 2019
We are excited to announce a partnership with CTMC, a joint @IncResilience & @MDAndersonNews venture, to accelerate novel cell therapy delivery. 🙌. We are looking forward to working together to enable widespread patient access to cell therapies. ➡️
0
0
11
Current #technology for CGT manufacturing isn’t fit for purpose. Join our webinar with @ElevateBio on Aug 6, 11 AM ET, to learn how early tech integration accelerates scalable, quality-driven manufacturing to meet patient demand. Register:
event.on24.com
Wednesday, August 06, 2025 at 11:00 AM Eastern Daylight Time.
0
0
0
With manual labor estimated to account for 40-50% of total CGT #Manufacturing costs, automation is key to immediate cost reduction. That’s why we built IRO®. Learn how automation drives cost reduction in our Whitepaper➡️
0
0
0
If you’re thinking about CGT scale in Phase 2, you’re already behind. Henrik Andersen, former Head of Cell Therapy Tech Dev at BMS, joins @Ori_JCFoster to talk about building commercial success from day one. 🎧 Listen to the Ori Spotlight Podcast:
0
0
0
Webinar: Join us and @elevatebio to learn how automated, closed-system manufacturing is reshaping CGT manufacturing. 💬 'Accelerating Cell & Gene Therapy Scale-Up With An Advanced Manufacturing Platform'.📆 Aug 6, 2025.⏰ 11:00AM ET.📍 Online. Register:
0
0
0
Cost of goods analysis reveals that CGT #Manufacturing contributes up to 50% of total costs. Automation is the first step to addressing manufacturing inefficiencies and improving the accessibility of CGTs. Read our Whitepaper to learn more ⬇️
0
0
0
Bioprocessing platforms that enable flexibility and scalability are critical to meet patient demand for CGTs. With @GENbio, @Ori_JCFoster discussed how we’re partnering with best-of-breed providers and technologies to expand flexibility for developers⬇️.
1
0
0
🔬 @abbvie's $2.1B acquisition of @capstantx highlights continued momentum for in vivo CAR-T. By reprogramming T cells inside the body using mRNA-LNPs, the approach could sidestep manufacturing bottlenecks and make CAR-T more accessible. Read more:
0
1
1
Capital-intensive manufacturing with low yields continues to challenge CGT delivery. @AKAarsalan, Founder of @endpts, joined @Ori_JCFoster on the Ori Spotlight Podcast to discuss how rethinking manufacturing will allow the industry to move forward. 🎧
0
0
1
Jennifer Le’s lupus diagnosis in 2016 brought ER visits, fatigue, and concerns about being able to start a family. In 2023, she joined a CAR-T trial and 6 months later, she’s back at work, and planning for the future. 🔗
www.nytimes.com
Lupus can be debilitating and sometimes deadly for the 3 million people who have it. A treatment called CAR T appears to stop it in its tracks.
0
0
1
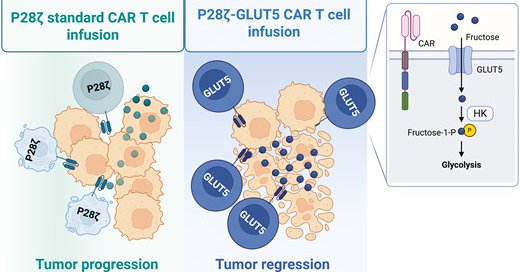
Researchers at @KingsCollegeLon have engineered CAR-T cells to use fructose when glucose is scarce in solid tumors. This modification preserves anti-tumor activity which could help improve CAR-T efficacy against solid tumors. 🔗
academic.oup.com
AbstractBackground. Cancer immunotherapy with engineered T cells has become a standard treatment for certain haematological cancers. However, clinical tria
0
0
0
📊 One of CGT’s biggest hurdles isn’t science. It’s economics. @endpts founder @AKAarsalan joins @Ori_JCFoster on the Ori Spotlight Podcast to talk investor sentiment, stalled assets, and why execution will define CGT’s next chapter. Listen now:.🎧
0
0
2
In our webinar with @CGT_Insights, our CCO @Thomas_Heathman and Senior Scientist Hamza Patel, explored how fluid dynamics can accelerate CGT development and support closed, scalable manufacturing from process development to commercial scale. Watch here:
0
0
0
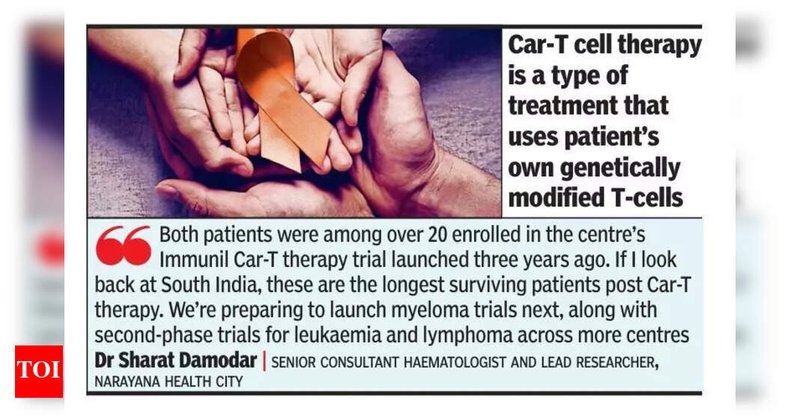
Rehan and Parimala are South India’s longest-known CAR-T survivors. Treated at @NarayanaHealth after years of failed therapies, both are now in remission and rebuilding their life. A reminder of what’s possible when CGTs are truly accessible. 🔗
timesofindia.indiatimes.com
Rehan and Parimala, two South Indian cancer survivors, are thriving after Car-T cell therapy at Narayana Health City. Rehan, in remission from lymphom
0
0
1
Developers need #Manufacturing solutions that evolve with the science, support rapid iteration & scale across therapy portfolios. That’s why we built IRO® – a flexible, modular platform to support development from R&D through to GMP. Read our Whitepaper➡️
0
0
0
🎙️ Our CEO @Ori_JCFoster is taking to the stage for @somXhealthtech's Healthtech Talks with @googlecloud, where he'll join a panel exploring the role of #tech in enabling patient impact at scale. 📆 June 30, 18:30 BST.📍London. Register your interest:
0
0
0