Moffitt Laboratory
@MoffittLab
Followers
678
Following
29
Media
42
Statuses
61
A research group passionate about developing genomic microscopy methods to illuminate microbe-host interactions. @BostonChildrens @harvardmed | moffittlab.bsky
Boston, MA
Joined April 2023
Excited to share our new work from Rosalind Xu, where we used MERFISH to build a single-cell spatial transcriptomic atlas of the mouse gut—and to explore host-microbiome interactions in situ! https://t.co/PFcrFPx8ih 1/
4
16
80
Taken together, these results underscore the potential of our new murine gut atlas to inform a broad range of biological and biomedical questions! 12/12
0
0
1
Remarkably, many aspects of the gut were largely unchanged! Nonetheless, we observed profound shifts in immune cell abundance and distribution—highlighting the central role of the microbiota in shaping and educating the immune system. 11/
1
0
0
Finally, while the microbiota shapes nearly every aspect of host physiology, its impact on the molecular, cellular, and spatial structure of the gut remained unclear. To address this, we rebuilt our entire atlas using mice lacking a microbiota. 10/
1
0
0
The gut microbiota produces metabolites that impact host immunity and even CNS function—but how? Using a near-comprehensive receptor panel, we mapped metabolite sensitivity across gut cell types, revealing rich hypotheses for cell mediated microbiota–host interactions! 9/
1
0
0
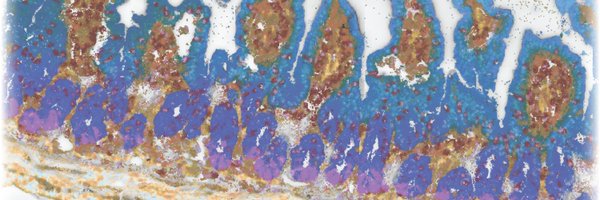
We also found a surprising set of genes with spatially patchy expression! In a great collaboration with Phillip Nicol (Irizarry lab), we developed a method to detect these patches —revealing insights into interferon signaling and epithelial specialization near GALT! 8/
1
0
0
This mucosal ruler revealed that nearly all cell populations fine-tune their gene expression based on their micron-scale position in the mucosa. Importantly, once one knows what to look for, these spatial signatures are also detectable in single-cell RNA-seq data! 7/
1
0
0
Another surprise was the intricate spatial organization of cells and RNAs in the mucosa. From crypt base to lumen, we observed gradients in cell types and gene expression, some known, many novel! We created an expression-based mucosal spatial ruler to capture this pattern. 6/
1
1
1
Our atlas revealed multiple surprises! For instance, we found a novel mature enterocyte marked by Scnn1g, a subunit of the epithelial sodium channel, and the pseudogene Best4-ps —suggesting it might be the long-sought mouse homolog of the human BEST4+ enterocyte! 5/
1
0
0
Excitingly, these advances enabled us to define a remarkable diversity of cell populations—78 in total—with finely resolved subdivisions within immune cells, fibroblasts, and the enteric nervous system! 4/
1
0
0
As small molecule receptors are often lowly expressed even when functional, we developed new approaches to improve the sensitivity of our MERFISH measurements, including an improved cell segmentation pipeline. 3/
1
0
1
The mammalian gut is home to a massive diversity of microbe-, diet-, and host-derived small molecules. To provide new insights into the cellular and spatial organization of sensation, we profiled 2.1M cells across four gut regions and two microbiome states! 2/
1
0
1
Yesterday our first grad student -- DR. Rosalind Xu -- had her official PhD hooding ceremony in @HarvardCCB! A massive congratulations to her and all of the other freshly minted PhDs!
2
0
31
As plasma cells play fundamental roles in tissue behavior in health and disease, we are excited to see the wide range of questions that BCR-MERFISH will now allow us and others to address! 9/9
0
0
2
To our surprise, we found that some plasma cell clones form patches of co-occurring clones along the gut, suggesting, perhaps, that there is local production of IgA specific to a subset of the microbiome! 8/9
1
0
4
Plasma cells modulate the microbiome by secreting IgA, and different plasma cell clones secrete IgA that targets different bacteria. But how these cells are distributed along the gut is not clear. Excitingly, BCR-MERFISH can now measure this distribution. 7/9
1
0
2
Importantly, BCR-MERFISH labels not only the Clone ID, but also the transcriptome of all surrounding cell types in the same slice, placing unique clones within their tissue contexts with single-cell resolution! 6/9
1
0
2
To test BCR-MERFISH, we profiled V gene usage in plasma B cells in the mouse ileum, defined B cell clones via the VH and VKVL gene choice within individual B cells, and showed that the usage of these genes agreed with BCR-sequencing. 5/9
1
0
2
However, MERFISH has been unable to discriminate B cells based on the BCR sequence because of the high degree of homology between V, D, and J genes. Evan solved this problem with a homology-aware probe design and encoding approach to produce a technique we call BCR-MERFISH! 4/9
1
0
2
However, some cell types - such as plasma B cells – may only differ in their expression of a single transcript, the B cell receptor (BCR). Yet, it is the sequence of this receptor, generated through V(D)J recombination, that sets their antigen specificity! 3/9
1
0
2