MeiHongLab
@MeiHongLab
Followers
661
Following
4
Media
17
Statuses
42
Our lab develops and applies magic-angle-spinning solid-state NMR spectroscopy to elucidate the structure and dynamics of biological macromolecules.
Cambridge, MA
Joined December 2019
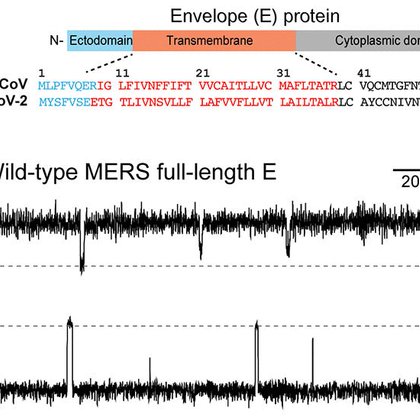
Check out our new study of the structure and ion channel activity of the MERS envelope protein!
science.org
The MERS E protein conducts K+ ions using aromatic residues.
0
0
10
Solid-state 19F NMR reveals the binding site of a PET ligand in tau fibrils adopting the Alzheimer's disease fold!
pubs.acs.org
Aggregation of the tau protein into cross-β amyloid fibrils is a hallmark of Alzheimer’s disease (AD) and many other neurodegenerative disorders. Developing small molecules that bind these tau...
2
3
29
Turn old photos into videos and see friends and family come to life. Try Grok Imagine, free for a limited time.
730
1K
5K
See our just published Account of our virus membrane protein NMR research!
pubs.acs.org
ConspectusEnveloped viruses encode ion-conducting pores that permeabilize the host cell membranes and mediate the budding of new viruses. These viroporins are some of the essential membrane proteins...
0
4
25
Our new study of how tau fibrils with the Alzheimer’s disease structure bind lipid membranes just came out at JACS! Check it out here
0
3
15
Check out our new NMR study of how a dynamic polar network in the SARS-CoV-2 E protein mediates proton and calcium ion binding!
0
2
17
See Joao giving fun science lectures to local school kids in the Azores!!
rtp.pt
Apresentação | Vera Santos - Açores Hoje aborda temas regionais, com reportagens de todas as ilhas dos Açores e com a presença regular de convida
0
0
4
Two structures of tau fibrils containing phosphorylation mimetics give new insights into the phosphorylation code of tau.
0
5
28
A tau construct (297-391) reported to replicate the Alzheimer’s paired helical filament structure instead forms non-twisting fibrils under high MgCl2 concentrations. Our atomic-resolution ssNMR structure reveals how this structure is stabilized. DOI: 10.1073/pnas.2310067120
0
0
13
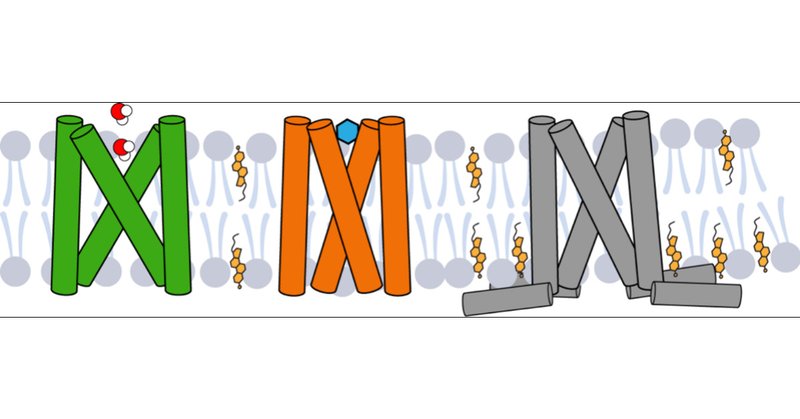
Using solid-state NMR, we solved the open structure of the SARS-CoV-2 E viroporin. Compared to the closed state, the open state has a wider N-terminal pore and a tighter C-terminal region, suggesting the cation conduction mechanism of this channel. DOI: 10.1126/sciadv.adi9007
0
2
32
Many Hong lab members attended the Protein Society Meeting here in Boston this week. It was an excellent meeting, with great science and great interactions.
0
0
20
Check out our new study that shows high-curvature cholesterol-containing membranes induce tau fibrils!
0
10
39
19F NMR data shed light on how three phenylalanine residues in the envelope protein of the SARS-CoV-2 virus controls the opening of this cation channel.
The Envelope Protein of SARS-CoV-2 is a viroporin that may induce severe inflammation for COVID. ssNMR+19F gave us the structure and an aromatic gating mechanism regulating this viroporin (💊🎯), induced by calcium and acidic pH. @MeiHongLab #GlobalnmrTC2022 +poster below
0
1
17
Summer volleyball at MIT chemistry, here by the team of "magic angels"!
0
1
7
Solid-state NMR data reveal that the tau protein associates with microtubules through a pseudorepeat domain R', while a proline-rich region and an R1 domain remain mobile.
1
12
93
Jammy plants! See how impaired methylation of pectins lead to dwarf plants because of significant changes in the cell wall structures, as detected by solid-state NMR
0
2
19
The small methyl group can do big things! Solid-state NMR reveals how de-methylation of pectins in plant cell walls dramatically affect wall structure and slow plant growth.
0
2
22
Solid-state NMR data reveal that two versions of the protein tau are fluently mixed in the neurotoxic tangles in the Alzheimer brain, defining the structure of the third dimension of these fibrils.
1
15
93
19F NMR illuminates how acidic pH and calcium ions change the sidechain conformation of two aromatic residues in the SARS-CoV-2 envelope protein to permit cation conduction.
1
8
103
Second structure of EmrE determined by NMR, finally showing this transporter in action, using pH-controlled alternating access to pump out toxic compounds! .
nature.com
Nature Communications - EmrE transporter effluxes cationic substrates across lipid membranes in a pH-coupled manner. Here, the authors solve the structure of ligand-bound EmrE at high pH by NMR,...
1
6
29