Janika Schmitt
@JanikaSchmitt
Followers
267
Following
324
Media
5
Statuses
56
Biosecurity & biotech governance at Sentinel Bio
Washington, D.C.
Joined March 2022
So excited to be joining Sentinel Bio! Couldn't ask for better teammates in our shared mission to make pandemics history within our lifetimes. Grateful for the warm welcome @jtmonrad Find us at https://t.co/kFIn8MPZ2V!
And then they were 3! Welcome to the team @JanikaSchmitt — we’ve definitely thrown you right into the deep end, but after a couple of weeks together in DC, Amsterdam, and Brussels, I’m very confident we’ve landed a rockstar for our small but mighty impact machine at Sentinel
0
0
12
In 2024, @ClaireQureshi and I went from a moonshot idea to founding Sentinel Bio and raising >$10M in philanthropic capital for pandemic prevention. Reflecting on our progress so far and the road ahead, my primary feeling is relentless optimism.
1
4
33
IMO there is little persuasive writing on why it's worth working on preventing worst-case pandemics. But this report by Founders Pledge from p 64 onwards summarises the case pretty well. https://t.co/5fBpxc6fhi
1
1
5
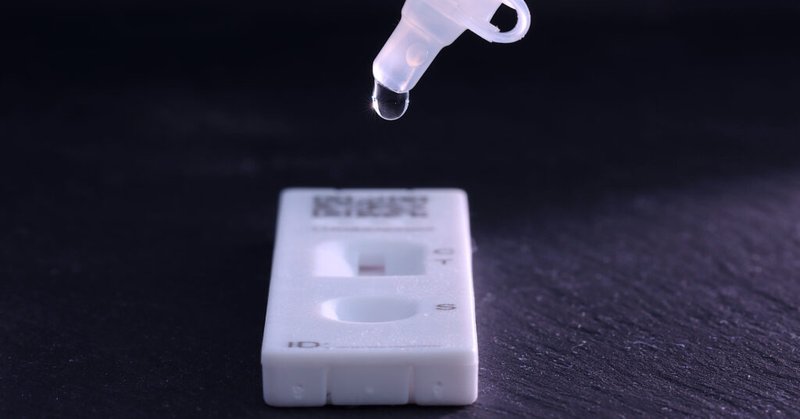
Love the specificity of these policy ideas. It's crazy to me we don't already have tests you can take at home and reconfigure quickly for new pathogens. This is absolutely an achievable goal. But diagnostic markets are tough to build stable businesses in. Public $/support needed
Rapid at-home tests suffer from - chronic underinvestments - uncertain demand - regulatory red tape It doesn’t have to be that way. For @IFP, @jacobswett and I do the deep dive on 5 policy ideas for developing 'triple rapid tests' for outbreak control 🧵:
3
11
94
Grateful to everyone involved, esp. all experts who took the time to talk to us! Read the full piece here:
ifp.org
Rapid reconfiguration, rapid deployment, and rapid results could help contain future outbreaks at their source
0
0
2
5) The FDA should streamline regulatory approval to evaluate TRF tests based on their public health benefits, considering factors like rapid results, accessibility, and scalability next to traditional accuracy metrics
1
0
2
4) ASPR should prioritize an agile and reconfigurable Strategic National Stockpile model to ensure the rapid availability of tests during emergencies — regardless of the pathogen in question. An agile SNS model would also help keep a warm manufacturing base
1
0
4
3) ASPR and CMS should foster sustainable demand through pre-purchase agreements and long-term reimbursement, reducing market uncertainty for the private sector.
1
0
4
2) Congress should establish RADx as a permanently funded entity within NIH. RADx has been crucial in accelerating rapid OTC test development and approval during COVID-19, but it's now at risk of being defunded
1
0
3
1) US funding agencies should support R&D and tech transition of TRF tests. We need a broad portfolio spanning detection methods like antigen tests, nucleic acid amplification tests, and array-based tests, as well as different sample types like saliva, blood, or breath
1
0
2
But: many companies that received US taxpayer money during the pandemic now face a deadly combination of regulatory uncertainty and lack of demand. To achieve TRF tests, we need a coordinated effort involving multiple USG agencies and the private sector. Here are our key recs:
1
0
2
Here, we introduce the Triple Rapid Framework (TRF) for outbreak control. TRF tests prioritize three essential features: 1. Rapid reconfiguration 2. Rapid deployment 3. Rapid results. The TRF aims to deploy 10,000 at-home tests within 10 days of outbreak detection
1
0
3
For instance, in early 2020, South Korea swiftly implemented widespread testing and contact tracing, flattening the epidemic curve at a manageable social cost. They tested earlier and much more widely than the U.S.
1
0
3
We can do better. Instead of accepting month-long development timelines as inevitable, we should aim to deploy tests within *days* of detecting an outbreak. Early & widespread testing could potentially prevent widespread transmission.
1
0
4
https://t.co/6kY8IxPlMR In an era of mRNA vaccines, we shouldn’t rely on quarantines for people with unknown infection status. Yet the U.S. got its first COVID at-home test 8 months into the pandemic, and we still don’t have rapid tests that specifically test for H5N1.
ifp.org
Rapid reconfiguration, rapid deployment, and rapid results could help contain future outbreaks at their source
1
0
7
Rapid at-home tests suffer from - chronic underinvestments - uncertain demand - regulatory red tape It doesn’t have to be that way. For @IFP, @jacobswett and I do the deep dive on 5 policy ideas for developing 'triple rapid tests' for outbreak control 🧵:
1
12
59
Find the full article here: https://t.co/WCgC1LQh9D
statnews.com
Opinion: We need to adapt tests as tools for quick outbreak control.
0
0
2