Guided Solutions
@GuidedSolutions
Followers
724
Following
118
Media
691
Statuses
5K
Guided Solutions is the Global Executive Search & Selection partner to the Medical Device industry.
Joined February 2012
With $27M in total funding, Subsense is accelerating next-gen R&D in nanoparticle sensing, biosafety, and hardware miniaturization, bringing safe, scalable, and high-fidelity BCIs closer to real-world use. 👉 Full news: https://t.co/CtVJkixHwd
#MedTech #NeuroTech #DigitalHealth
news.gsmedtech.com
Medical Device News by Guided Solutions | Subsense, a developer of non-surgical invasive, nanoparticle-based brain-computer interfaces (BCIs), has added $10 million in new capital following
0
0
0
🧠 Clinical evidence continues to build - and now the #FDA has granted De Novo approval for @Neurovalens' Modius Lean platform. 🔍 Read more: https://t.co/255NAIhrhb
#MedTech #MedicalDevices #MedicalEquipment #Neurotechnology
news.gsmedtech.com
Medical Device News by Guided Solutions | Neurovalens, the Belfast-based neuro-technology company, has been granted approval by the US Food & Drug Administration (FDA) for Modius Lean, its
0
0
0
FDA clears @HeartBeamInc's at-home 12-lead ECG system. Wallet-sized device, cloud-based analysis, cardiologist follow-up - bringing arrhythmia monitoring into patients’ homes. 🎉 🔎 Full story: https://t.co/g50gnTBxEw
#MedTech #Cardiology #ECG #Arrhythmia #DigitalHealth
news.gsmedtech.com
Medical Device News by Guided Solutions | HeartBeam has secured US Food and Drug Administration (FDA) clearance for its 12-lead electrocardiogram (ECG) synthesis software, setting the arrhythmia
0
0
0
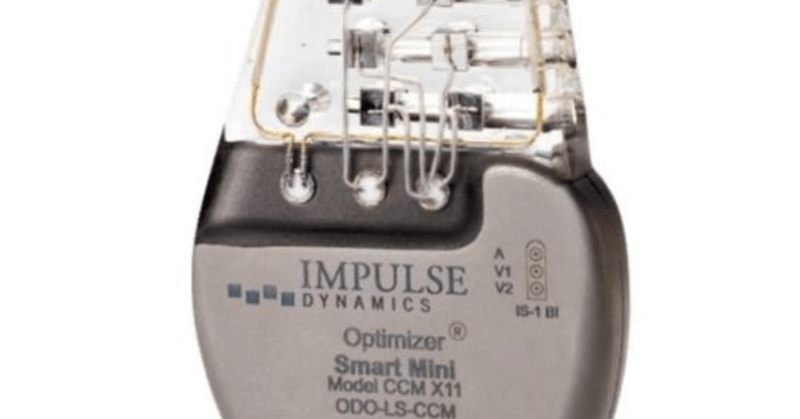
$158M for @ModulateHF signals real momentum in HF care! With CMS coverage now in place, Impulse Dynamics' CCM therapy is set to reach far more patients. 🧡 🔗 Full story: https://t.co/DY3nP65cLj
#MedTech #MedicalDevices #HeartFailure #Cardiology #CardiacCare
news.gsmedtech.com
Medical Device News by Guided Solutions | Impulse Dynamics announced that it raised more than $158 million in financing to support its heart failure treatment technology. New institutional
0
0
0
With a $150M ASX IPO, @SaludaMedical is now scaling its commercial and R&D efforts, driving the next era of precision neuromodulation. 🧠 📰 Get the full details: https://t.co/6wdFQtw8ag
#MedTech #MedicalDevices #Neuromodulation #ChronicPain
news.gsmedtech.com
Medical Device News by Guided Solutions | Saluda Medical announced that it raised $150 million through an initial public offering on the Australian Securities Exchange (ASX). Minneapolis-based
0
0
1
#MedTech @ROCAMED_SAM and @Delmont_Imaging are reshaping the future of minimally invasive Uro-Gyn care, by combining Rocamed’s expertise with Delmont’s technologies! 👉 https://t.co/3nqVDeCxMi
#MedicalDevices #Urology #Gynecology
0
0
0
#MedTech @Resmed’s Smart Comfort is now FDA-cleared - the first AI-enabled system to recommend personalized CPAP settings for obstructive sleep apnea. Using real-world sleep data, it helps users adjust therapy comfortably.💡 🔗 https://t.co/dFyuGExnxo
#DigitalHealth #SleepApnea
news.gsmedtech.com
Medical Device News by Guided Solutions | Resmed announced that it received FDA clearance for its Smart Comfort personalized therapy offering. San Diego–based Resmed calls Smart Comfort (which was
0
0
0
📢 The ePORE electroporation platform is moving closer to clinical reality with €7.2M funding for PIONEER - @Mirai_Medical’s next-generation program aimed at transforming colorectal cancer therapy. 🔗 Learn more: https://t.co/NkzpzpHuUu
#MedTech #HealthTech #Oncology
news.gsmedtech.com
Medical Device News by Guided Solutions | Mirai Medical, the lead applicant and coordinating partner, has been awarded €7.2M under the Disruptive Technologies Innovation Fund (DTIF) Call 7 for
0
0
0
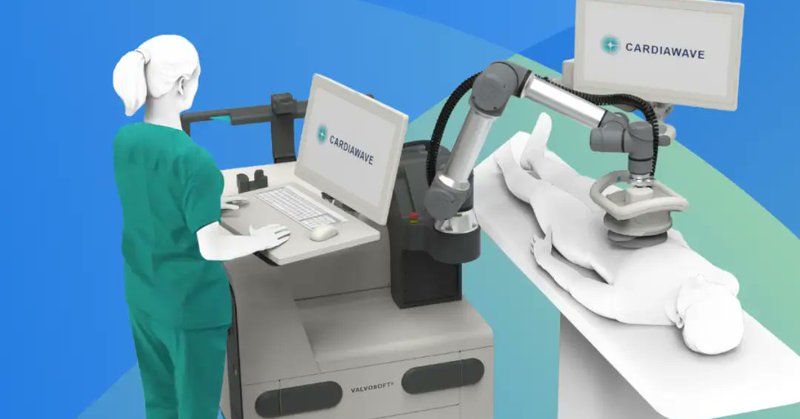
#MedTech @Cardiawave won #CEMark for Valvosoft: a device that uses therapeutic ultrasound pulses to soften and repair calcified aortic heart valves. EU market entry is on the horizon. 🎯 https://t.co/gr0iSWO0lf
#MedicalDevices #MedicalEquipment #HealthTech #MedTechNews #MedTech
news.gsmedtech.com
Medical Device News by Guided Solutions | French MedTech Cardiawave has gained an EU CE mark for its non-invasive ultrasound therapy (NIUT) Valvosoft, marking the first device of its kind
0
0
0
China-based Tuodao Medical launched with a multi-specialty #surgicalrobotics platform. Unlike competitors that focus on a narrow niche, its wide scope can set it apart in a busy market. 👉 https://t.co/qt4kiTYNyr
#MedicalDevices #MedicalEquipment #MedTechNews #MedTech
news.gsmedtech.com
Medical Device News by Guided Solutions | Tuodao Medical announced its launch as a developer of robotic solutions for intelligent medical care. The China-based company wants to make surgery easier
0
0
0
#MedTech @AtraverseInc wins #FDA 510(k) for HOTWIRE - a fully-integrated, zero-exchange RF guidewire and generator system that simplifies safe, sheath-agnostic left-heart access. 👉 https://t.co/7GdDn0Gx17
#MedicalDevices #MedicalEquipment #HealthTech #MedTechNews #MedTech
news.gsmedtech.com
Medical Device News by Guided Solutions | Atraverse Medical announced that it received FDA clearance for its fully integrated Hotwire transseptal access system. Hotwire is a novel radiofrequency
0
0
0
A $138m upsized stock offering will accelerate @KestraMedical's commercialisation and scale-up of its Assure wearable cardioverter defibrillator for patients with a weak heart. 🔗 https://t.co/xuatR3wMfe
#MedicalDevices #MedicalEquipment #HealthTech #MedTechNews #MedTech
massdevice.com
Kestra Medical Technologies (Nasdaq:KMTS) announced that it priced an upsized underwritten offering worth approximately $138 million.
0
0
0
The Vanquish water-vapor system from @FrancisMedical gained #FDA 510(k) clearance for prostate cancer ablation, with the VAPOR 2 data showing very positive clinical results. 🔗 https://t.co/ypfDljoOU5
#MedicalDevices #MedicalEquipment #HealthTech #MedTechNews #MedTech
news.gsmedtech.com
Medical Device News by Guided Solutions | Francis Medical’s Vanquish device for treating intermediate-risk prostate cancer has been cleared by the US Food and Drug Administration (FDA). Vanquish
0
0
0
A new #Partnership in #Orthopaedics: Shoulder Innovations & @INSRobotics team up to build a shoulder-specific micro-robotic platform - portable, OR-ready, and ASC-friendly. 🔗 https://t.co/DIk1cCYIDj
#MedicalDevices #MedicalEquipment #HealthTech #MedTechNews #MedTech
news.gsmedtech.com
Medical Device News by Guided Solutions | Shoulder Innovations announced a strategic partnership with Interventional Systems on a shoulder-specific micro-robotic solution. The companies plan to
0
0
0
#Cardiac #MedTech @VisCardiaInc raised $40M to push its VisONE diaphragmatic-stimulation heart failure therapy into pivotal U.S. trial, aiming for first implant in early 2026. 🔗 https://t.co/9GwYWOK3qT
#MedicalDevices #MedicalEquipment #HealthTech #MedTechNews #Cardiology
news.gsmedtech.com
Medical Device News by Guided Solutions | VisCardia, a medical device company pioneering a novel cardiac-support therapy for patients with heart failure, announced it has secured $40 million in
0
0
0
Avandra acquires DatCard & Sorna to build a global, end-to-end medical imaging platform, combining clinical data sharing and research-grade imaging data aggregation under one roof. 🔎 https://t.co/SuMF5onMCW
#MedicalDevices #MedicalEquipment #HealthTech #MedTechNews #MedTech
news.gsmedtech.com
Medical Device News by Guided Solutions | Avandra Health has acquired DatCard Systems and Sorna Corporation for creating a medical imaging platform aimed at supporting patient care. The move
0
0
0
Led by CEO Elaine Schutte, @Xeltis raised €47.5M from @EIB and existing shareholders to advance regenerative vascular access graft #aXess to market. Trial data is very positive. 👉 https://t.co/xPx2JCCQ28
#MedicalDevices #MedicalEquipment #HealthTech #MedTechNews #MedTech
news.gsmedtech.com
Medical Device News by Guided Solutions | Spurred by a positive clinical trial readout recently, Xeltis has raised €47.5m ($55.1m) to advance commercialisation efforts for aXess, a vascular access
0
0
0
$53M in Series C #funding will support @Akura_medical accelerate development of its Katana thrombectomy system + NavIQ software. The goal - enable data-driven clot removal. 📰 https://t.co/TEXTVD1umJ
#MedicalDevices #MedicalEquipment #HealthTech #MedTechNews #MedTech
news.gsmedtech.com
Medical Device News by Guided Solutions | Akura Medical, a Shifamed portfolio company, announced that it completed the first close of its Series C financing, raising $53 million. Los Gatos,
0
0
0
A new #CEMark for @ArtiriaM's SmartGUIDE 014 #neurovascular guidewire. With its small size - 0.014 in - it enables improved navigation through the brain’s challenging vasculature. 🔗 https://t.co/56hpjerJn8
#MedicalDevices #MedicalEquipment #HealthTech #MedTechNews #MedTech
news.gsmedtech.com
Medical Device News by Guided Solutions | Artiria Medical, a Swiss innovator in neurovascular technologies, proudly announces that its SmartGUIDE 014 deflectable guidewire, a next-generation
0
0
0
#MedTech @a2zradiologyai wins FDA clearance for a2z-Unified-Triage - an #AI tool that flags & prioritises 7 urgent findings on abdomen–pelvis #CTscans in a single pass, promising faster emergency triage in #radiology. 🔎 https://t.co/Dh84LCuwSh
#MedicalDevices #MedicalEquipment
news.gsmedtech.com
Medical Device News by Guided Solutions | 2z Radiology AI has secured approval from the US Food and Drug Administration (FDA) for its a2z-Unified-Triage, a multi-condition triage system for
0
0
0