Francesco Cortiula
@FCortiula
Followers
356
Following
677
Media
3
Statuses
298
Medical Oncologist- #lungcancer #thoracic #oncology #protontherapy @UdineUniversityHospital PhD candidate @MaastrichtU @MaastrichtUMC @MAASTRO
Udine, Italy
Joined March 2020
Can we de-escalate ICIs in NSCLC?💡 Are we ready to reduce financial and patients' tox?🔎 Honoured to work on this topic with terrific colleagues @HendriksLizza @JordiRemon @JessicaMenis @g_mountzios @BenjaminBesseMD @MarianaBrandao0 @NReguart @MartinaBortolot
De-Escalation Strategies With ICIs in NSCLC: Do We Already Have Enough Evidence? @JCO_ASCO Pleased to contribute to this great review led by @JordiRemon @GustaveRoussy @OncoAlert @g_mountzios @HendriksLizza @FCortiula @JessicaMenis @MarianaBrandao0 #LCSM
0
5
13
Italian National Team at #WCLC2025 @AriMarinel @RobertoFerrara_ @Max_Cani @Gmazzaschi90 @pecci_federica1 @doctordegio @CarloGenova5 and many others 🇮🇹🍕
1
12
29
Discuss a wide range of case studies with leading experts! Access practical cancer IO insights at @sitcancer’s #virtual ACI: A Focus on Lung Cancers program Sept. 12. View topics: https://t.co/7aGu6hKmR5
@ChaftJamie @FCortiula @DrJNaidoo @drshieldsmd
#LearnACI
0
4
6
Press release updates data for NVL-520, zidesamtinib, in #ROS1 NSCLC. In 117 pts who had prior TKI (half had 2+ TKIs), RR 44% with 78% ongoing after one year. Intracranial efficacy and active in ROS1 G2032R. Tolerability: discontinuation rate only 2%! https://t.co/jshs9bMRSD
investors.nuvalent.com
Aligned with FDA on NDA submission strategy for TKI pre-treated patients with advanced ROS1-positive NSCLC and participation in Real-Time Oncology Review; the company plans to initiate a rolling...
0
17
51
📣Updated ESMO #ClinicalPracticeGuideline recommendations in oncogene-addicted metastatic #NSCLC: more #TargetedTherapy agents now available in 1L or at resistance. #LungCancer #mNSCLC #ESMOGuidelines 🔗 https://t.co/Ns4jjSTo3e
0
15
28
📣Updated ESMO #ClinicalPracticeGuideline recommendations in the non-oncogene-addicted metastatic #NSCLC: new 1L Tx options approved. 🔗 https://t.co/S04MDA13Jn
#LungCancer #mNSCLC #ESMOGuidelines
0
17
37
#ASCO25 Developmental Therapeutics CSS🔥 Ph III DELLI trial: ultra-low dose nivo in 2L all solid tumors v chemo @VanitaNoronha @TataMemorial: - 500pts - OS HR 0.80 (p=0.02, NS primary endpt HR 0.75) - no diff in PFS/ORR/tox A brave trial, next step evaluate chemoIO? @asco
0
5
19
Why is CM816 so important? After @FordePatrick beautiful presentation of these revolutionary data, let’s dig into why this unique trial should shake up our vision for oncology research…
5
33
104
#ASCO25 Dr. @charlesrudin presents interim analysis of DeLLphi-304: randomized phase II study of tarlatamab (DLL3 TCE) vs 2L chemo in #SCLC. Chemo was mostly topotecan; 45% of pts were platinum resistant. Clear OS benefit with HR 0.60 (13.6m vs 8.3m). PFS 4.2 vs 3.7m, HR 0.71.
6
39
118
Fascinating paper @NatureCancer on mechanisms of resistance to TIL in NSCLC: - serial multiomic analysis, 16pt TIL trial - subclonal neoAg lost at progression - LOH of pMHC in KRAS-specific Tcells at progression @BenCreelan @MoffittNews @OncoAlert #LCSM
https://t.co/p7jYl5l546
2
21
69
In @JTOonline #BRAF-mutated #NSCLC: https://t.co/mo2dvSVXSJ 📊 1L targeted therapy (dabrafenib/trametinib) vs. chemo-IO shows: ➖ Similar OS outcomes ➖ PD-L1 status & gender influence prognosis 🎯 Personalization > one-size-fits-all @LungCancerRx @oncodaily @OncBrothers
2
12
45
Congrats to all the authors for this great collaborative effort🏆 ICI retreatment after durvalumab for relapsed stage III NSCLC should be offered to selected patients💥📢 These results also confirm our previous findings🎯 @HendriksLizza
@AndrearicFili
https://t.co/UMmzRt3Gz3
ejcancer.com
The current standard of care for fit patients with unresectable stage III NSCLC involves concurrent chemoradiation (CRT) followed by durvalumab. Disease recurrence occurs in approximately 2/3 of...
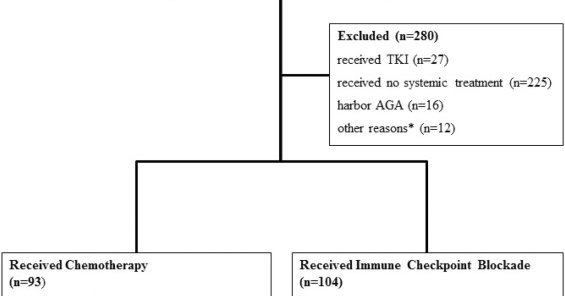
Real world outcomes in pts with relapse after durvalumab consolidation post CRT for stage III NSCLC @LungCaJournal. With chemo-immunotherapy, PFS 12m; with platinum-based chemo alone, PFS 4.1m; non-platinum based chemo PFS 2.7m; targeted therapy PFS 6m. https://t.co/ffVX9For3h
0
3
9
Personalised approach at osimertinib PD with savolitinib and osimertinib in MET deregulated EGFRm NSCLC looks promising. Is it better than CT+Ami or ADC? Hwr, it is a chemofree strategy and other treatments could be applied later #ELCC25
0
35
79
We had efficacy data ADCs after osi-PD in EGFRm NSCLC. Today good mPFS (9.5-11.7) with Osi+DatopotamAb Dxd after osi-PD. Data looks ➕promising than chemo+Amiv. Better 🧠 protection maintaining osi? Biomarker for dato? Toxicity is imp. Future trial: osi-DATOD vs Ami-CT? #ELCC25
0
32
110
🔥European Journal of Cancer🆙 ✅Efficacy of Immunotherapy retreatment vs Chemo in Unresectable NSCLC with Progression Post CRT and Durvalumab 🎯PFS/OS HR 0.67 (95%CI 0.49-0.91) / 0.61 (0.43-0.86). ICB might offer⤴️survival 👥@FCortiula
#LCSM @OncoAlert
https://t.co/2m1X7TEuPD
ejcancer.com
The current standard of care for fit patients with unresectable stage III NSCLC involves concurrent chemoradiation (CRT) followed by durvalumab. Disease recurrence occurs in approximately 2/3 of...
0
10
23
Immunotherapy-based treatment versus Chemotherapy-only in Patients with Unresectable NSCLC with Disease Progression Post Chemoradiation and Durvalumab - European Journal of Cancer
ejcancer.com
The current standard of care for fit patients with unresectable stage III NSCLC involves concurrent chemoradiation (CRT) followed by durvalumab. Disease recurrence occurs in approximately 2/3 of...
0
9
30
🚨 ICB Retreatment in unresectable Stage III #lcsm: Who Benefits? 🔎 ✅ PFS ≥12m on durvalumab? ICB retreatment improved OS (22m vs. 9.8m with chemo, p=0.024). ❌ PFS <12m? No survival benefit with ICB vs. chemo—huge unmet need! 🧩❓post-durvalumab space! https://t.co/JABuRv0cLv
2
19
51
📣 Just updated: ESMO Non-oncogene-addicted mNSCLC Living Guideline v1.2 👉Find new recommendations with links to ESMO-MCBS scores, easily accessible from desktop and mobile devices. #ESMOGuidelines 🔗 https://t.co/MbK3tqKL4E
0
11
28
IO has changed ttx and outcome in adv 🫁.It’s time that Academic trials (PULSE @BenjaminBesseMD )and international grants test IO de-escalation to ensure ➕ pts receive these drugs, ⬆️outcomes to more pts worldwide, while ⬇️financial tox.Thk all authors!👏🏻 https://t.co/ez3S7YQ56i
0
24
56