Addict
@Biopharmaddict
Followers
2,340
Following
714
Media
186
Statuses
579
Explore trending content on Musk Viewer
DAVI NO DOMINGAO
• 210387 Tweets
Flamengo
• 169874 Tweets
Corinthians
• 126054 Tweets
Knicks
• 88318 Tweets
Wesley
• 84669 Tweets
Brunson
• 75779 Tweets
Embiid
• 74502 Tweets
Dallas
• 54432 Tweets
Luka
• 48308 Tweets
Sixers
• 43917 Tweets
Sporting
• 41372 Tweets
Clippers
• 40764 Tweets
Kyrie
• 38665 Tweets
Velez
• 31825 Tweets
Sevilla
• 31333 Tweets
Mavs
• 28924 Tweets
Felipe Melo
• 25248 Tweets
Betis
• 23086 Tweets
LOBOS COM AMSTEL
• 22611 Tweets
Diniz
• 22145 Tweets
Harden
• 22061 Tweets
昭和の日
• 20990 Tweets
Kawhi
• 17174 Tweets
76ers
• 16377 Tweets
Paul George
• 15345 Tweets
Mavericks
• 15259 Tweets
Cano
• 15103 Tweets
Renato Augusto
• 11952 Tweets
Lowry
• 10632 Tweets
Last Seen Profiles
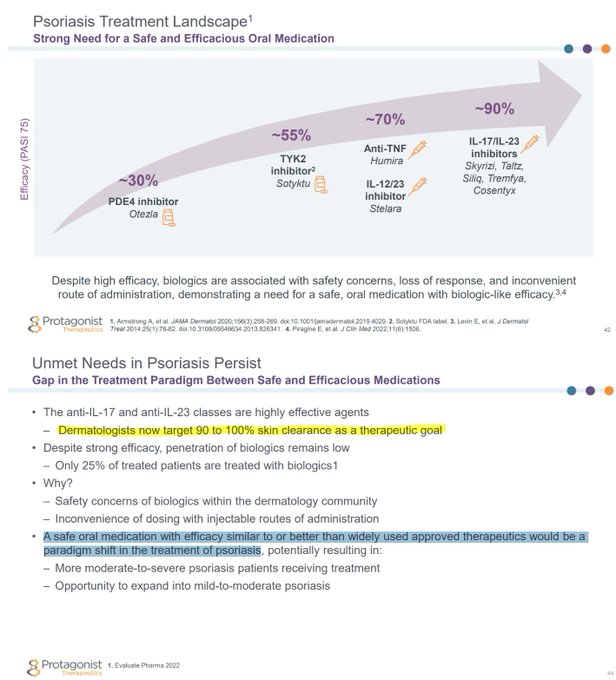
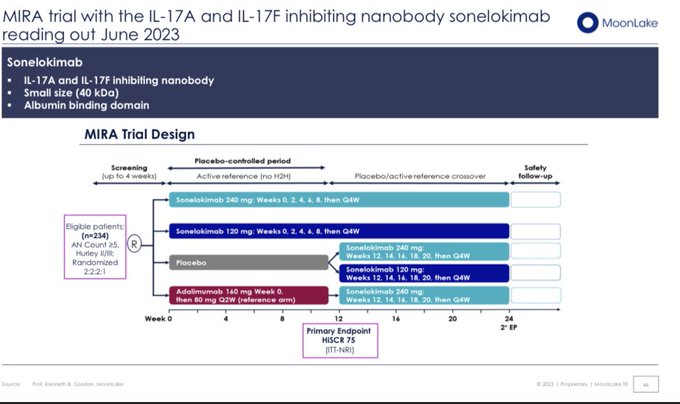
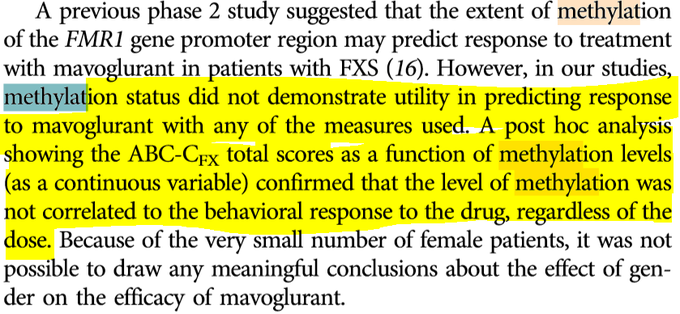
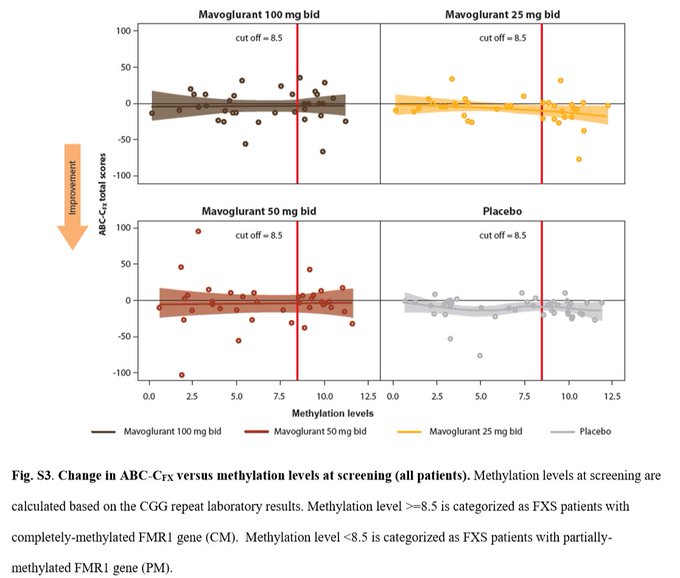
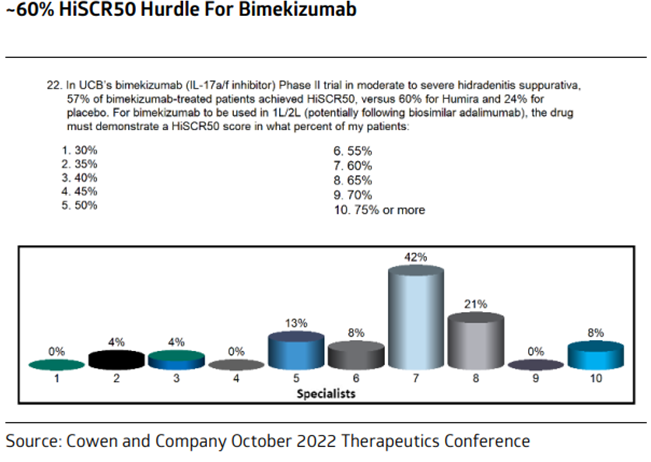
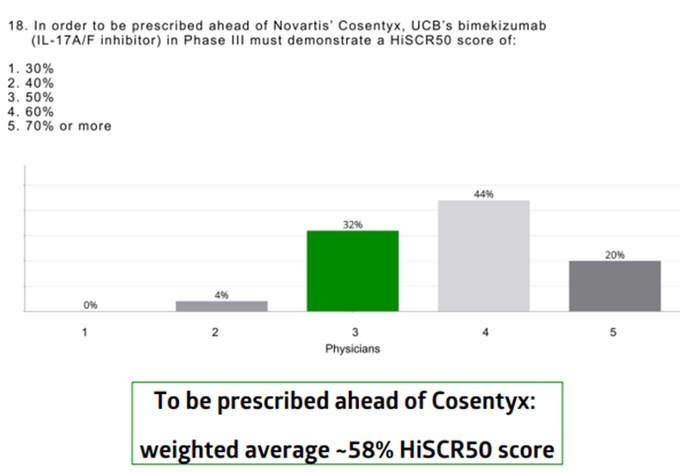
Worst case scenario for $MLTX's SLK (IL-17A/F nanobody) at
#AAD2023
this weekend:
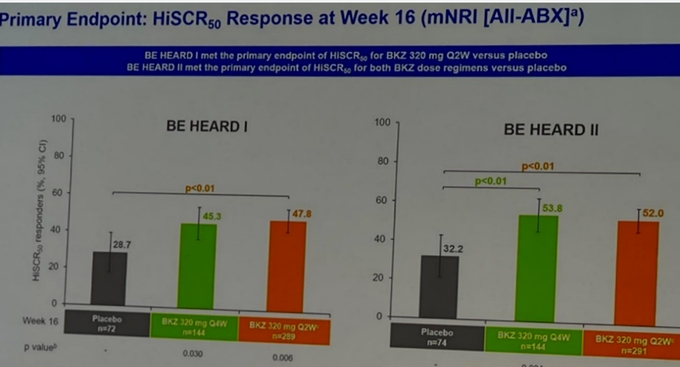
BIMZELX (IL-17A/F mAb) significantly underwhelmed vs expectations in HS on HiSCR50/HiSCR75.
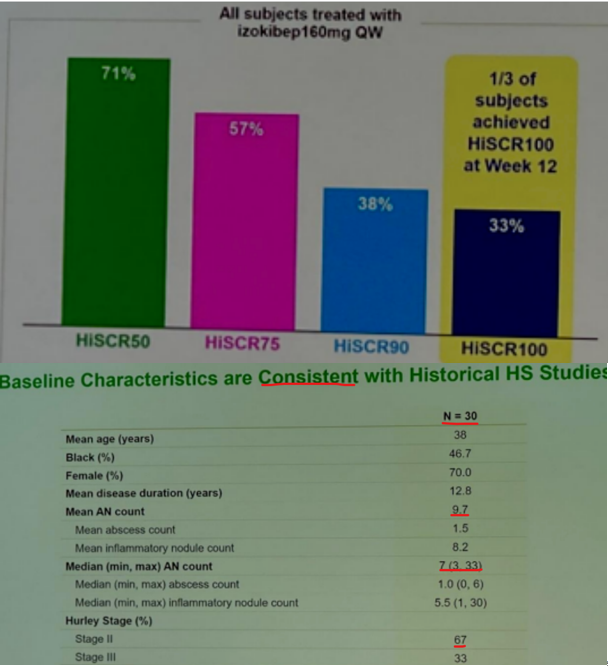
izokibep (IL-17A small kDa mAb) results are ambiguous due to more mild patients enrolled.
2
6
46
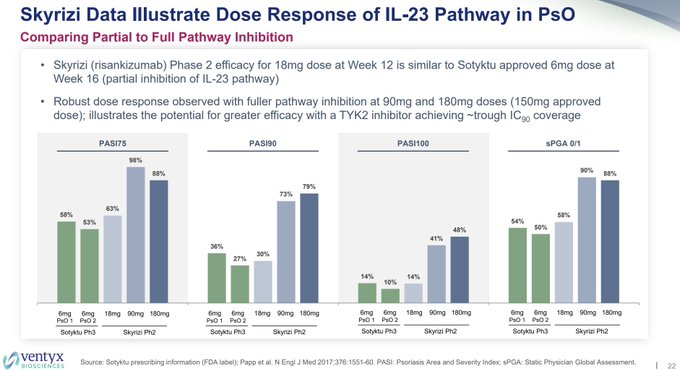
Checking in on the hot new nanobody companies we have:
$SLRN
HS, HiSCR75
❌160mg QW failed vs pbo
❌160mg Q2W failed vs pbo
$MLTX
PsO, PASI100
❌30mg failed vs COSENTYX
❌60mg failed vs COSENTYX
❌120mg failed vs COSENTYX
❌120mg w/ LD failed vs COSENTYX
PsA, ACR50
❌60mg NI…
Worst case scenario for $MLTX's SLK (IL-17A/F nanobody) at
#AAD2023
this weekend:
BIMZELX (IL-17A/F mAb) significantly underwhelmed vs expectations in HS on HiSCR50/HiSCR75.
izokibep (IL-17A small kDa mAb) results are ambiguous due to more mild patients enrolled.
2
6
46
0
6
33
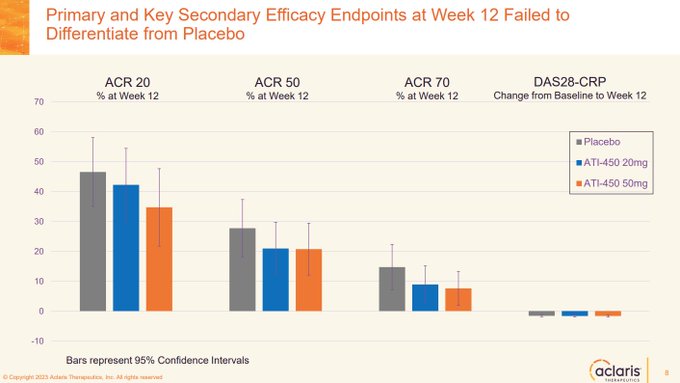
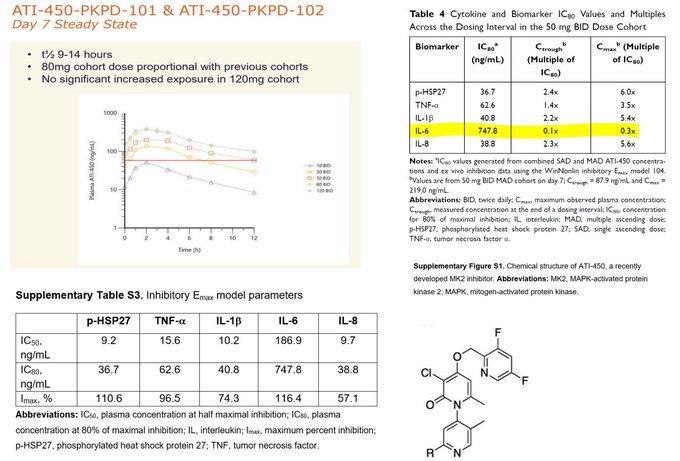
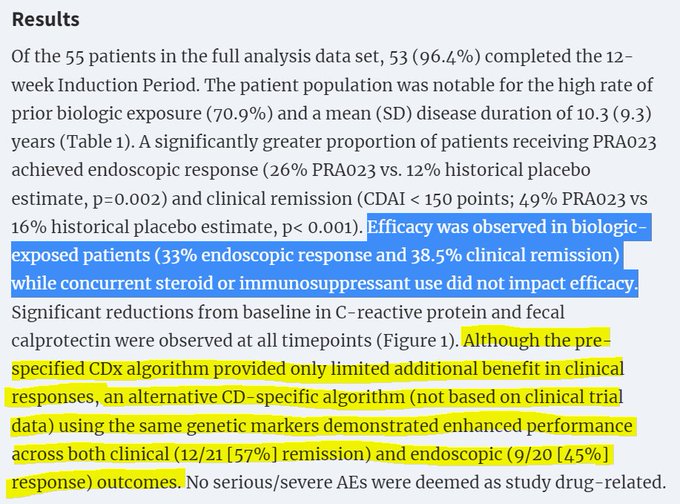
$ACRS "There was no notable differentiation between zunsemetinib and placebo across any measures of efficacy at week 12."
$ACRS enjoys the unique pleasure of their drug performing *worse* than placebo.
Might be because their drug was a weak cytokine inhibitor with minimal TNF-a…
4
5
24
$ELEV 38% ORR/57% DCR in all, 47% ORR/65% DCR in GC cohort
relatively clean safety for an ADC w/ only 24% AEs gr3+, 2 gr3 nausea and vomiting at 3 mpk
*at 11/5/2022 cutoff* - so more mature data at ASCO
MC ~$73MM w/ $74MM cash at 1Q23e. Comp to $AZN/Keymed $63MM uf + $1.1bn ms
$ELEV First-in-human dose escalation and expansion study of SYSA1801, an antibody-drug conjugate targeting claudin 18.2 in patients with resistant/refractory solid tumors.
#ASCO23
1
6
15
1
4
21
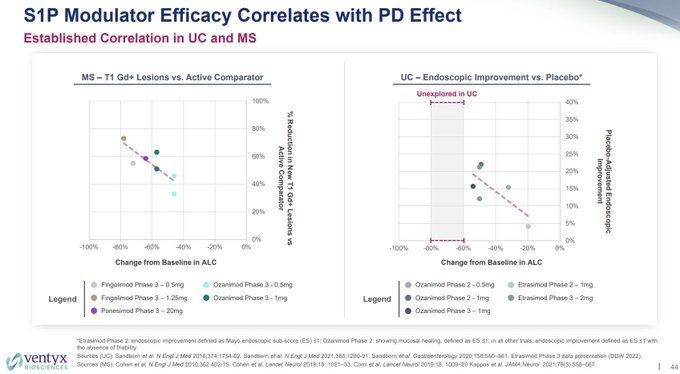
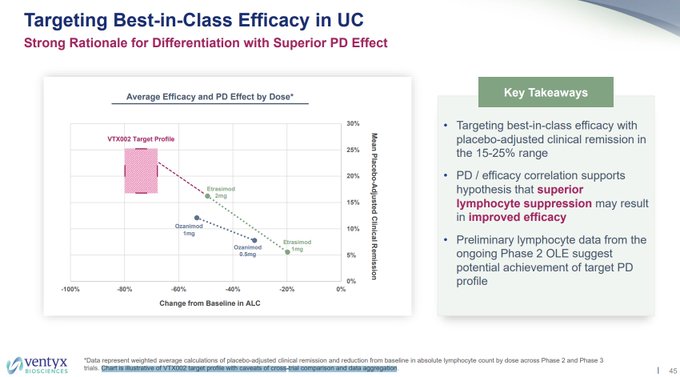
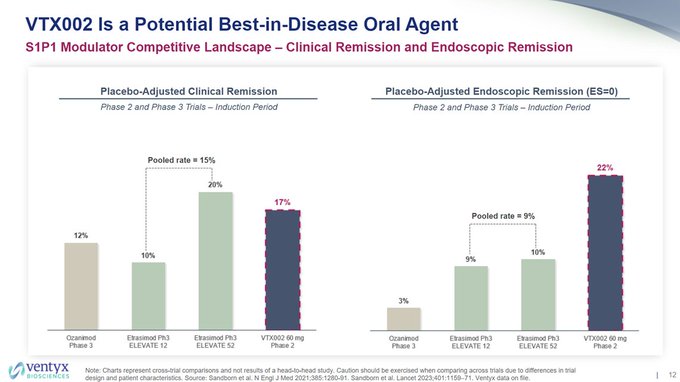
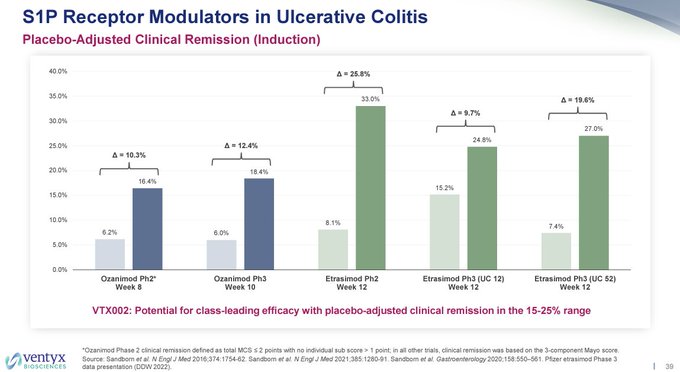
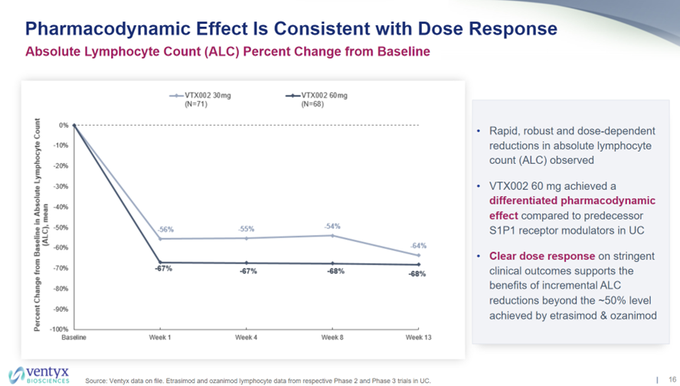
I wonder why $VTYX decided to remove the $ARNA phase 2 CR rate for etrasimod of ~26% pbo-adj for comparison to today's data, but noted it in their corporate deck 8/10, along with a bar of needing ~15-25% pbo-adj rate (sell side bar notes bar was 20-25%).
Also, so much for the…
0
3
19
Where were the signs? $VTYX
1
0
18
@jeromeleonard5
@buysidebio
No, not the same receptor occupancy - $PTGX is gut, $MORF is blood. $MORF is also using manganese-free assay to get most conservative look at Ctrough (many others don't - many times exaggerates RO). But mgmt guiding to >90% which is also consensus.
3
0
17
$APLS “[USPI now includes] the previously reported rare events of retinal vasculitis with or without occlusion in patients treated with SYFOVRE. The Company pursued this update in collaboration with the U.S. Food and Drug Administration.
The estimated rate of events of retinal…
1
3
17
If there's anyone who loves a thread from
@biotechinvstr
, it's me - one of the best around across the board, as I've often said privately.
IMHO, "body language" into data is driven more by standard sell side reining in expectations than an internal change of the bar at $RXDX
2
1
15
This is biotech gold. I wish all of my firesides were this good. “You may think we have 99 problems, I think we have none, but cash ain’t 1.”🔥 $TGTX
1
1
14
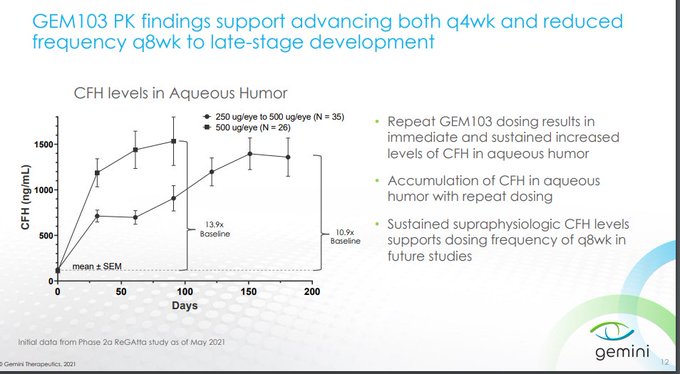
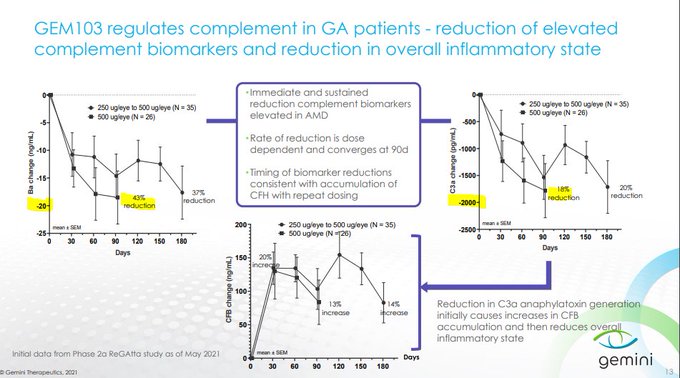
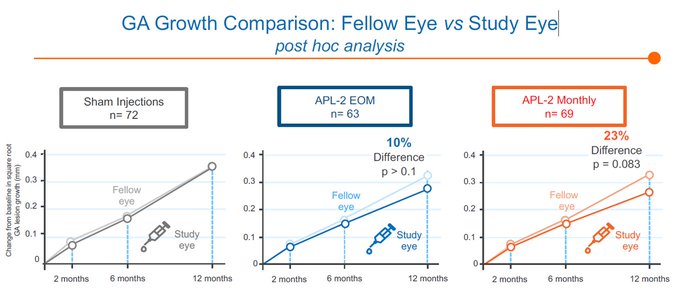
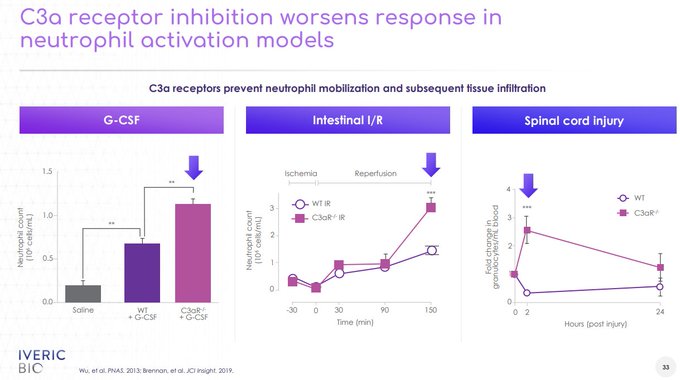
$GMTX confirms that supraphysiologic levels of CFH via GEM103 is unable to inhibit the complement cascade deep enough to bring patients to normal Ba/C3a levels, reflected in the lack of lesion growth separation from the contralateral eye
@sentivcapital
@coyotebioscav
@buysidebio
250ug/500ug isn't expected to show dose dependent biomarker reductions as drug concentrations are already so high...if dosed q4w up to 6 mos w/ minimal impact to biomarkers, how would that show GEM103 is active?
C3i's inhibit ~99% C3 with slowing of lesion growth at 2,3,6 mos
0
1
4
1
3
15
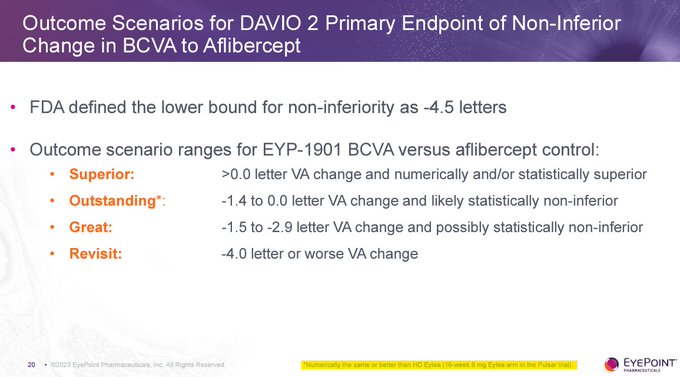
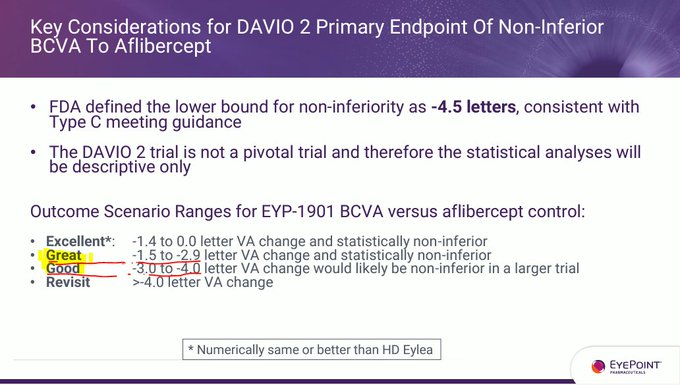
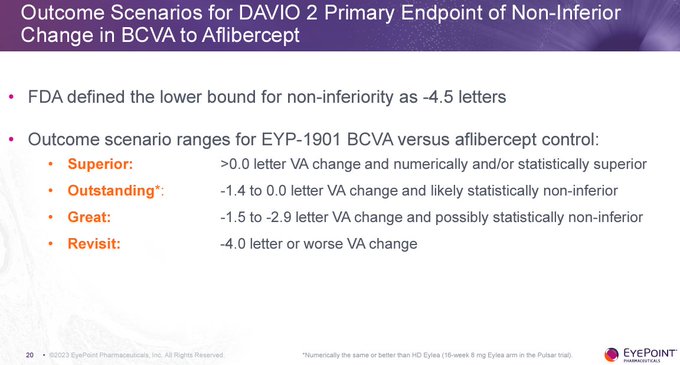
No - if $EYPT is not ≤ 2 letters vs 2mg EYLEA at 6 months then it's hardly different than 8mg EYLEA HD. This is reflected by $EYPT management updating the slide to note data from EYLEA HD in PULSAR.
But who cares about BCVA in phase 2 statistics - it's all about durability with…
0
1
10
This is entertaining…I remember my first three days digging into these studies for work on $TVTX with similar initial reactions before educating myself. $KDNY
1
0
10
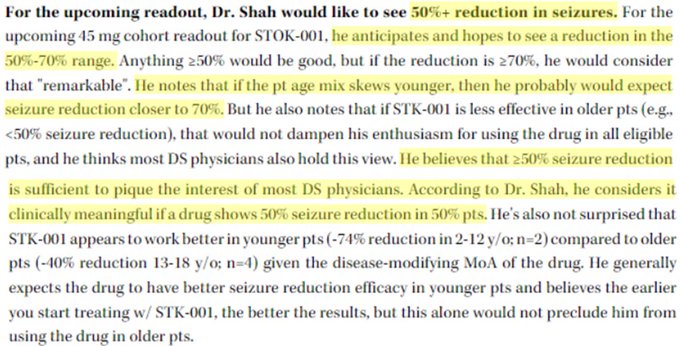
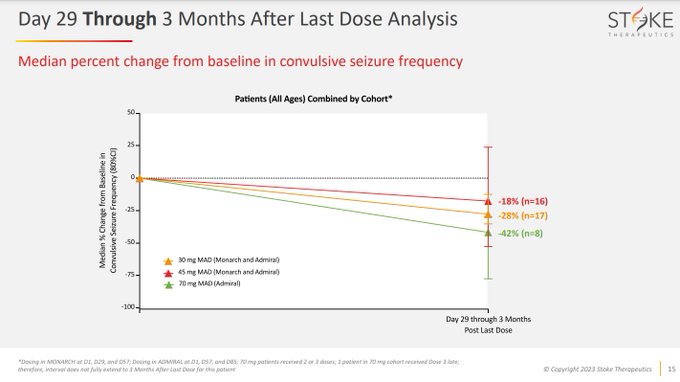
Why would 70mg be expected to be more effective when they're showing overlap with 45mg with N=8, knowing that data gets worse with higher N?
$STOK has maxed their PK/PD response with 30mg, 45mg didn't show improvements in PK/PD, so adding more drug without a PK/PD relationship…
2
0
10
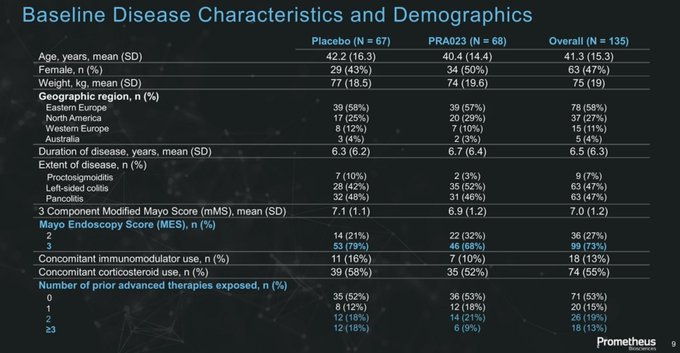
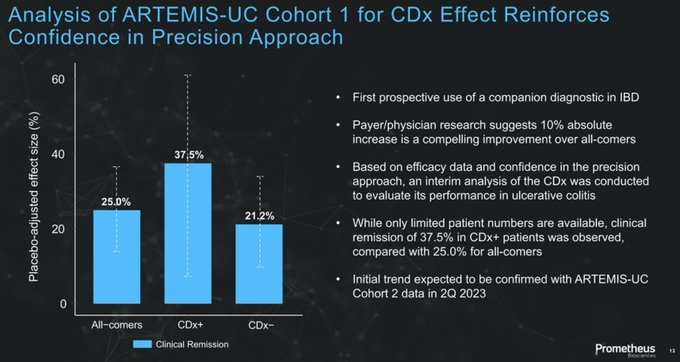
"RINVOQ-like efficacy with ENTYVIO-like safety."
Really exciting data from $RXDX with a drug that has dual potential to be first choice for both biologic naïve and experienced/failures as well as offer the 1st precision medicine approach for IBD patients
3
1
9
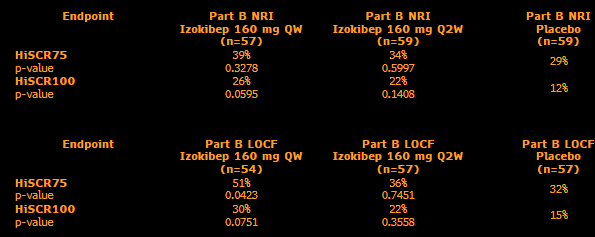
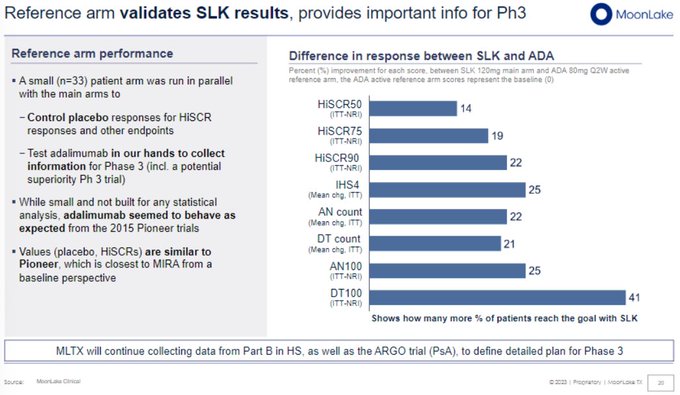
Ouch - confirmed this is relative difference, so whiffed on 10%+ delta over HUMIRA.
CANTOR: Humira arm performance in this trial was similar to its Phase 3 PIONEER-2 HS trial and showed ~36% HiSCR75 vs. SLK 43% (relative difference of 19%).
0
3
9
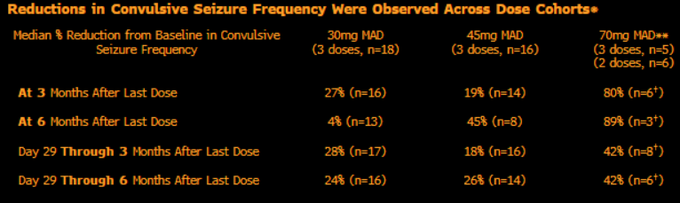
$STOK misses 45mg bar of >50% with only 19% seizure reduction going from N=6 to N=16.
Tries spinning with adding in 70mg data (was initially guided to 2H23) to save face - but even with 80% reduction in tiny N at 3 months, the day 29-3 month data is only -42% with highest N…
0
1
8
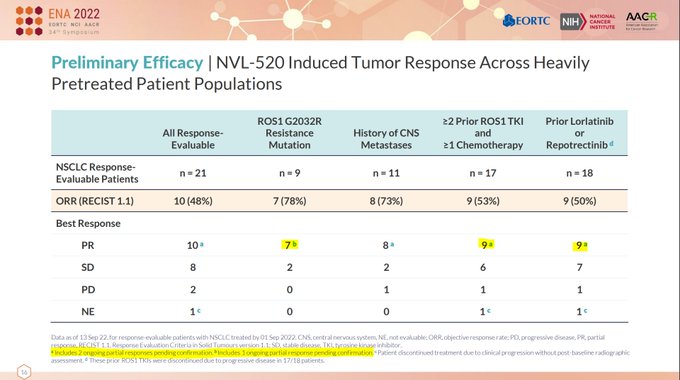
At >$1bn valuation, is $NUVL NVL-520 really differentiated from $TPTX repotrectinib and
@AnheartTx
taletrectinib, enough for stock to work?
Let’s see durability (not in PR??), safety table, *confirmed* ORR, and more specifics on patient characteristics…
1
1
8
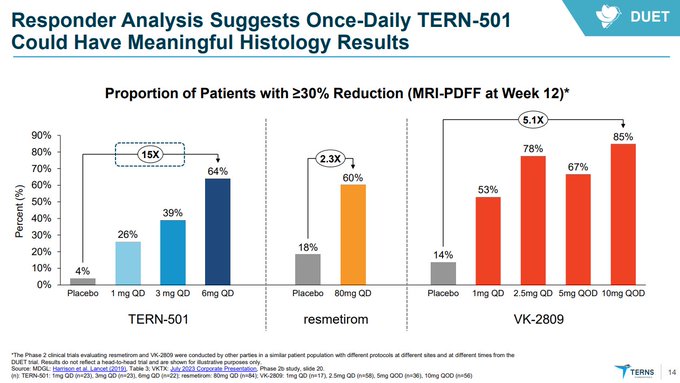
@bigpharmaguy
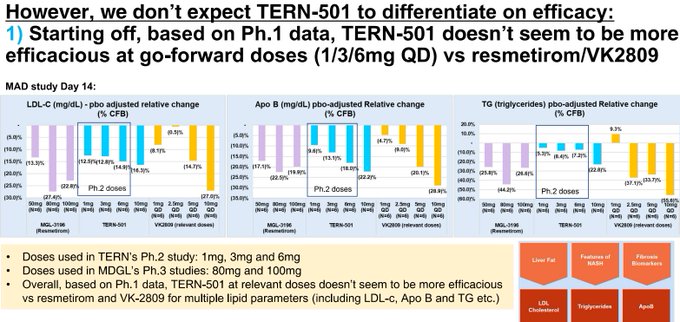
So $TERN is worse than $VKTX and undifferentiated-to-worse than $MDGL in ~1/4 the amount of patients trialed...how will this differentiate on the endpoints that matter in phase 3 reading out years from now?
1
0
7
@drug_smolecules
Some selling from tourists and no headline (if no $APLS announcement by open) but only ~5-10% max imho. It’s Astellas with their commercial and pipeline needs raising debt to buy, not $REGN $RHHBY $NVS with varying ophtho expertise placing a bet on the out of consensus winner.
2
0
7
In patients having moderate-to-severe itch at baseline, the seladelpar treated group improved their pruritus at 6 months compared to those in the placebo group (p<0.005) $CBAY
0
1
7
@plainyogurt21
@NoseRubInvest
@Sports_bios
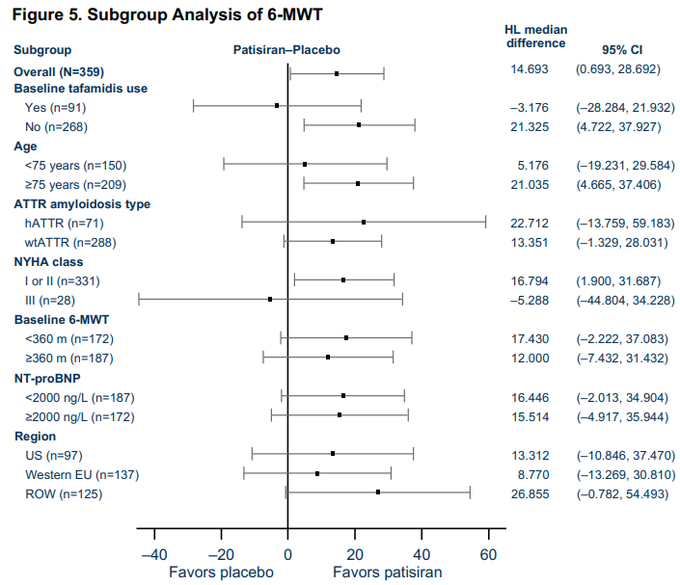
Odd that $BBIO's stated blended rate flattens out around 18 months while all others see acceleration in event rate despite tracking ATTR-ACT at 12 months...
And given NYHA Class I/II WT patients accounted for 40% of wins on deaths/40% of wins on # CVHs in ATTR-ACT and $BBIO…
2
0
5
Hey Europeans, especially Swedes, if your science is so good please lend your shares for us non-believers to short and risk getting our faces ripped off.
I guess I’ll reach out directly next time.
0
0
6
$RXDX additional data for the APOLLO-CD study in
#ECCO23
abstract compares nicely to the landscape.
1
0
6
Spot on - especially looking at the large difference in *trough* receptor occupancy achieved by PN-943:
100mg QD: ~25%
1000mg QD: ~79%
450mg BID: ~79%
@NoseRubInvest
@MSollender
I don't see any reason for this to have inverse dose response.
In previous Ph2a the company presented "a dose response".
0
0
5
0
0
6
@sentivcapital
@avidresearch
@bigpharmaguy
@Sports_bios
Given the history of how $RETA interacts w/ regulators, feigns transparency to the market regarding those interactions, and the black box surprises that came from bardoxolone BDs etc - even considering $AMLX corollary + nuances - less public exposure will increase nervousness
1
0
6
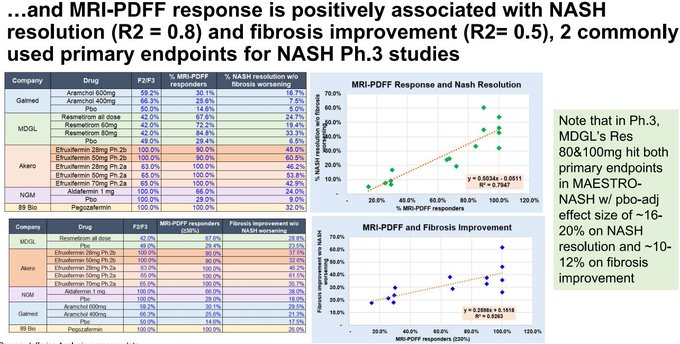
@bigpharmaguy
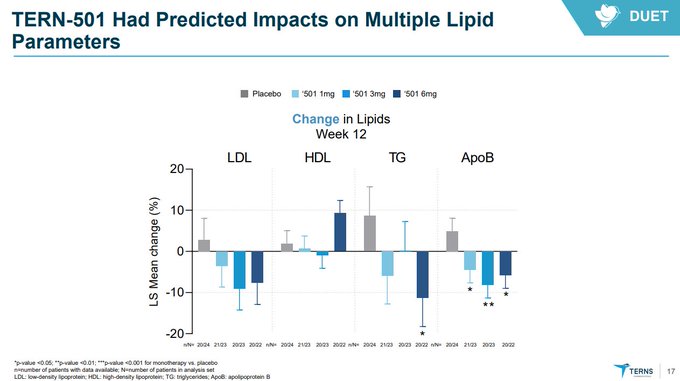
$TERN has worse effects on key biomarkers as well compared to $MDGL, $VKTX, and their own phase 1 data at 15 days versus 12 weeks here!
Differentiation thesis killed.
0
0
6
@biotechinvstr
@BiotechPort
This risk factor was added in 2017 10-K and hasn't changed since. Where is it said explicitly that both endpoints need to be SS for approval?
If you want a notable addition to a filing, see $ALBO's claim what is clinically meaningful *while they're in the midst of data clean up*
2
1
6
@AnheartTx
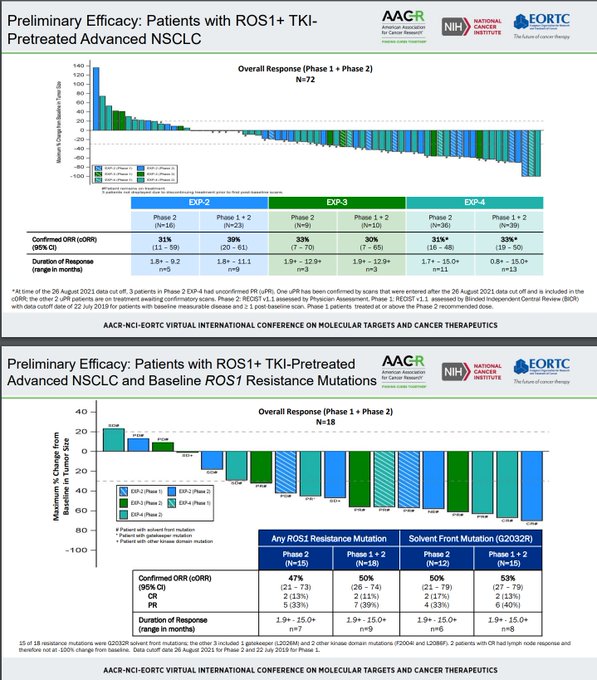
$NUVL cORR is 67% (6/9) in ROS1 muts and 38% (8/21) in all evaluable --> $TPTX degraded significantly from 50-60% in early phase 1 to ~30-35% in later phase 1/2 with larger N
1
0
5
@gcbioinv
@Biohazard3737
@Sanctuary_Bio
Endoscopies are centrally read, so the focus on “open-label” nature is null.
I get what you and many others are stating - it’s a good guide post that will save you a lot of money in most examples. But you’ve got to break the rules and go outside the guide posts in certain cases.
1
0
4
@BiotechPort
@ohadhammer
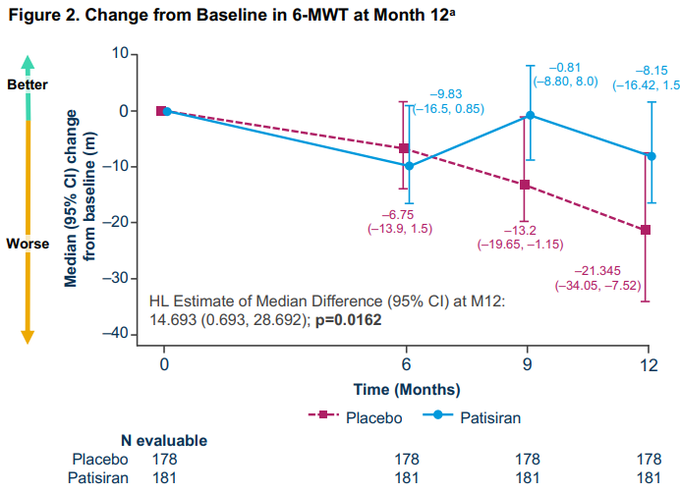
Superiority certainly not priced in given caution around 6MWD as an endpoint, no dose response with tafamidis, changes to inc/exc vs ATTR-ACT, etc…
0
0
5
@Biohazard3737
@gcbioinv
@Sanctuary_Bio
my brother in christ, ~90% of those patients were naïve to biologics vs ~30-40% have "advanced-therapy" experience (JAKs,S1Ps, etc) in $MORF
1
0
5