Jet Tsien
@JetTsien
Followers
87
Following
307
Media
1
Statuses
48
Joined July 2017
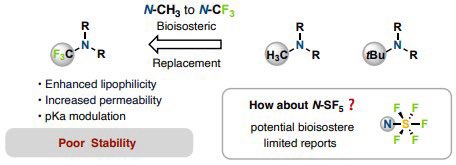
Congrats to the SF5 team @Renzhe1122 @Chaohu_103 in @QinLab . N-SF5 substituted azetidines really enlarged novel chemical space with SF5 substitution and will provide a new type of isosteres for medicinal chemists!.
If you are a medchemist, you must have considered introducing CF3 on amine to reduce basicity and improve ADME property, but gave up realizing its poor stability. Why don't you use SF5 now? Honored to be a part of this collaboration with @QinLab!.
0
0
3
Super excited to share this toolbox and it's really a great collaboration with all of my colleagues!.
💊Accelerating Medicinal Chemistry💊: A C(sp3)-rich Fragment Toolbox for Redox-Neutral Cross-Coupling, appearing today in @chemrxiv: A great collaboration with brilliant drug hunters at BMS, Pfizer, and Biogen. Quick summary: A new toolbox of 15 sulfonyl
0
2
15
This is a great collabotation with Dr. Renzhe Li @Renzhe1122 , Dr. Chang Liu, Dr. Chao Hu @Chaohu_103, @SijieChen17, @yuzururururu @rohanmerchant1 under the guidance and support from @QinLab. Kudos to Dr. Renzhe Li @Renzhe1122 for driving this amazing project.
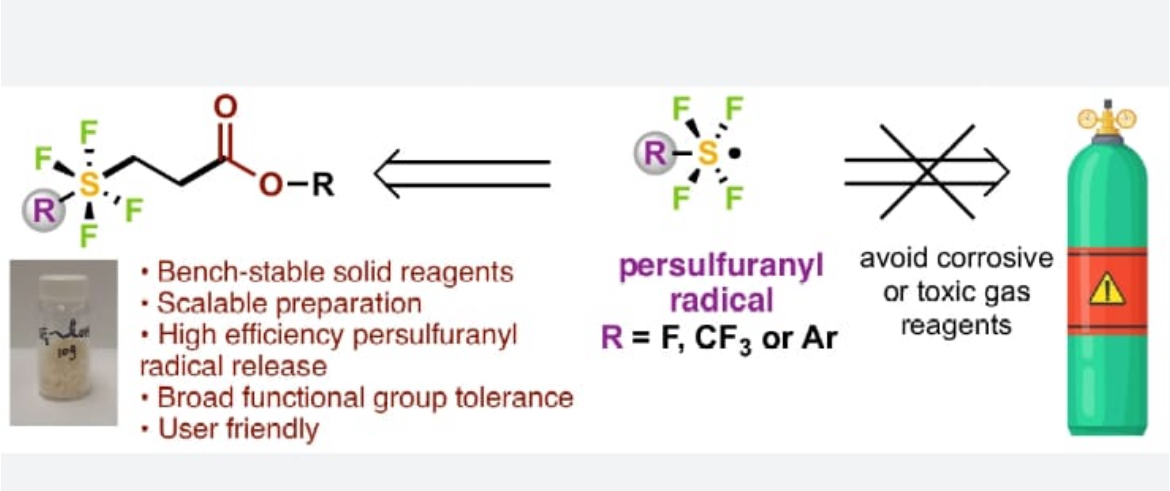
It is super excited to see one of my last projects in which I participated during my last months in UTSW come out: .Very excited to share our latest work on modular installation of persulfuranyls, SF5 in particular, with a series of bench-stable reagents.
0
0
3
So pleasant to participate in this amazing project and see it coming out!.
Finally, RADICAL CROSS COUPLING without the exogenous REDOX. We disclose in @ChemRxiv ( a broadly general platform for achieving transformations that normally required excess metallic or chemical reducing agents or photochemical setups (and their
0
0
10
RT @BaranLabReads: Finally, RADICAL CROSS COUPLING without the exogenous REDOX. We disclose in @ChemRxiv ( a broa….
0
140
0
RT @yuzururururu: The final version of this work has appeared today in @J_A_C_S .A General Three-Component Alkyl Petasis Boron–Mannich Reac….
0
17
0
RT @NatRevChem: Three-dimensional saturated C(sp3)-rich bioisosteres for benzene. A new review from @JetTsien, Cha….
0
16
0
RT @AsianJOrgChem: Reaction Paradigms that Leverage Cycloaddition and Ring Strain to Construction Bicyclic Aryl Bioisosteres from Bicyclo[1….
aces.onlinelibrary.wiley.com
In this minireview, we discuss different cycloaddition methods to access strained C(sp3)-rich bicyclic hydrocarbons, which are promising bioisosteres of aromatic rings. Each cycloaddition example is...
0
2
0
Very pleasant collaboration with our industry colleagues from Merck @rohanmerchant1 @SijieChen17 and Novartis @yuzururururu ! Really fantastic alkyl amine library via HTE implementation done by Si-Jie @SijieChen17.
Excited to share our recent collaboration with @QinLab to access highly functionalized sp3-rich amines via 3-component alkyl petasis reaction! Dump and stir aldehyde + amine + alkyl B(OH)2 + 🔦 #MerckChemistry @JetTsien @SijieChen17 @yuzururururu @ChemRxiv
0
0
7
RT @UTSWGradSchool: The deadline to apply for the SURF program this summer is around the corner! Don't miss your chance to learn from renow….
www.utsouthwestern.edu
UTSW's Summer Undergraduate Research Fellowship (SURF) program offers intensive training to college students who are preparing for biomedical research careers.
0
6
0
Cool!.
Selective P450BM3 Hydroxylation of Cyclobutylamine and Bicyclo[1.1.1]pentylamine Derivatives: Underpinning Synthetic Chemistry for Drug Discovery | JACS @UniofOxford @OxfordChemistry @Totalsynth #Hydroxylation #Cyclobutylamine #Biocatalysis #DrugDiscovery
0
0
0
RT @EdAndersonGroup: @EdAndersonGroup is delighted to share our Perspective on the beautiful BCP framework in @JACS_Au. Congratulations @Be….
pubs.acs.org
Bicyclo[1.1.1]pentanes (BCPs) have become established as attractive bioisosteres for para-substituted benzene rings in drug design. Conferring various beneficial properties compared with their...
0
12
0
RT @SarpongGroup: Congratulations to @B_randonwright and @iammjblack and our collaborators at Janssen on the publication of a skeletal edit….
0
13
0
RT @BaranLabReads: The final version of this work has appeared in @J_A_C_S today:
pubs.acs.org
A concise total synthesis of the complex guanidinium toxin KB343 is reported traversing through an unusual sequence of chemoselective transformations and strategic skeletal reorganization. The...
0
18
0
RT @ritter_lab: Check out our newest publication in @NatureSynthesis about O-, N- and C-bicyclopentylation using thianthrenium reagents!. h….
0
18
0
RT @HartwigGroup: Check out our latest @NatureChemistry paper on the catalytic undirected borylation of tertiary C–H bonds, done in collabo….
0
24
0
RT @MykhailiukChem: Is it BCP? Cubane? .Oh no, it is O-[2.2.2] - ideal Ph-bioisoster!.@ChemRxiv: .
0
30
0
RT @rohanmerchant1: Just in time for the holidays! A great collaboration with the @QinLab to escape the flatland by easily accessing more s….
pubs.acs.org
Heterocycles are the backbone of modern medical chemistry and drug development. The derivatization of “an olefin” inside aromatic rings represents an ideal approach to access functionalized saturated...
0
6
0